In the realm of material science, the composition of a material and a molecular level affects how a material will react to different forces, temperatures, and changes. Among the different material types are crystalline or crystal structures. A crystalline material composition contains smaller crystal structures whose molecules repeating patterns in every dimension. Each crystal structure is based on a unit cell pattern for which is repeating through the entirety of the material. In this article, you will learn about the different types of crystal structure, crystal structure properties, and manipulation of crystal structure.
Types of Crystal Structures
Crystal structures form off the shape of the unit cell within a crystal lattice. The unit cell represents the smallest unit that repeats in the entire structure of a crystal. Considering the unit cell is crucial to differentiate and identify the type of crystal structure a material is. Unit cell orientation is set on a three dimensional coordinate grid with the distance and angle from each axis being the difference. This section will cover the different types of crystal structures and the unit cell orientation that goes with it.
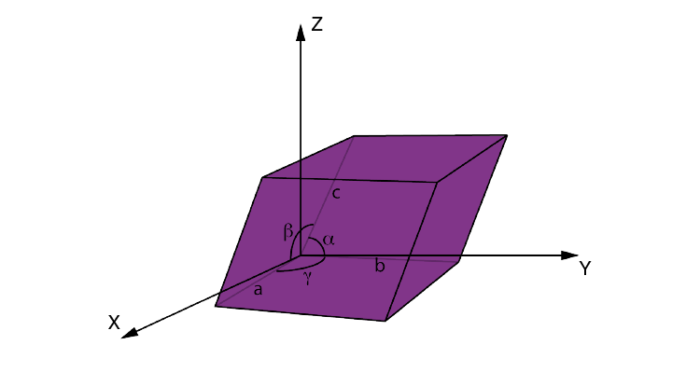
Primitive Unit Cells
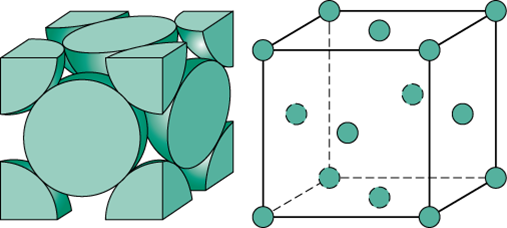
The primitive unit cell refers to a crystal cell in which molecules in only the corners of the unit cell shape. This is the most basic of the types of crystal structures. Also referred to as a simple cubic unit cell, a primitive unit cell has a single lattice point or atom per unit cell as each corner cell cuts each atom into an eighth.

Body Centered Unit Cell
Body centered unit cells, or BCC follows the same atom distribution of a simple cubic unit cell with an atom added to the center of the body. The atom in the centered added onto the simple unit cell structure results in a total of 2 atoms per unit cell.

Face Centered Unit Cell
Face centered unit cells of FCC is a unit cell in which atoms are in the center of the faces of a crystal unit cell as well as the corners of that in a simple unit cell. Because of this orientation, the atoms on each face are half an atom each. Combining all the atoms, the volume of a face centered unit cell comes out to 4 atoms per cell.
Crystal Structure Properties
For each lattice system there are different types of interplanar spacing within the crystal structure. The image below depicts the different types of lattice systems and the lengths and angles associated to each. From these numbers, different properties maybe identified to better reference different lattice systems.

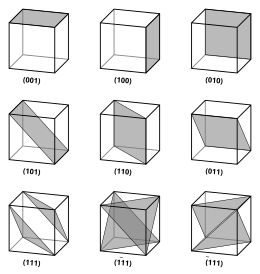
Miller Indices
Miller indices are notation forms for vectors and planes in a crystal lattice and are in the following format: (lmn) for which l, m, and n signify directions on the coordinate system of a crystal. A zero in place of an indices denotes that specific axis does not intersect the plane in question. A one in place of an indices denotes that specific axis does intersect the plane in question, and a barred 1 denotes a negative indices.
Interplanar Spacing
Interplanar spacing (d) is the spacing between adjacent planes and is specific to each different lattice structure. The formulas for the major shapes are as follows.

Manipulating Crystal Structures
Dealing with crystalline structures, you can manipulate different properties such as strength, ductility, and grain boundaries in a material. Because of this, grain structures form grain boundaries in a material. The orientation of these grains play a large role in how the material will react. Ideally, controlling the cooling of a hot material such as metal will result in larger crystals and grains such as heat treatment. When grains are larger in a material, the strength is lowered while ductility is increased. Whereas, quickly cooling a material leads to smaller crystals and a stronger less ductile material.
In metal specifically, cold working and strain hardening generate more grain boundaries which strengthen the material being altered. This prevents the existing boundaries from moving as more are introduced and created. Overworking a material this way will lead to an increase in brittleness in the long run. Annealing is a process in which crystals are heated to make larger ones and reduce boundaries. This process increases ductility and lowers strength.