Brittle materials break with little or no elongation prior to failure. In this article, you’ll receive examples of brittle materials, learn about fracture type, and determine how to reduce the unwanted brittle fracture.
Examples of Brittle Materials
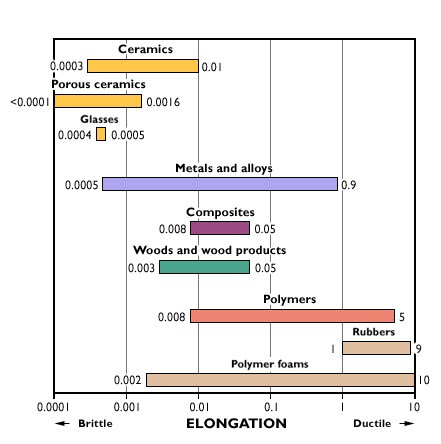
Materials that are commonly thought of as brittle include glass, ceramic, and specialty engineered alloys. Elongation provides a common measurement of ductility. Materials with 5% or less elongation prior to fracture generally are considered brittle.
Glass
Glass, a translucent, solid-like substance, sees various applications in our daily lives. Melting of natural and abundant raw materials such as sand, soda ash, and limestone at extremely high temperatures creates this new material.

At high temperatures, glass is structurally similar to liquids. However, at ambient temperature, it behaves like solids. As a result, glass can be poured, blown, pressed, and molded into plenty of shapes.
Glass is an example of brittle material due to its amorphous structure. There is no method to release stress in glass since there are no planes of atoms that can slip past each other. As a result, excessive pressure causes a fracture to form at a spot with a surface imperfection.
The tensile strength of glass is usually around 7 megapascals (1,000 psi). On the other hand, the theoretical upper bound on its power is orders of magnitude larger (17 gigapascals) (2,500,000 psi).
Ceramic
Another example of brittle material is ceramic. A ceramic is a complex, brittle, heat-resistant, and corrosion-resistant substance created by molding and firing an inorganic, nonmetallic material like clay at a high temperature. Earthenware, porcelain, and brick are common examples.

How does ceramic become brittle? Ceramic materials lack slip mechanisms that can distort them since they are polycrystalline structures of ionic or covalent connections. It is unavoidable to leave micro-defects on the material’s surface during the preparation process, leading to cracks.
Ceramics make it difficult for atoms to glide past one another. This is because ionic bonding dominates in ceramics, resulting in alternating positive and negative ions. If a row of atoms tries to slip beyond the next row of atoms, positive ions will gravitate toward positive ions, and negative ions will gravitate toward negative ions. From the standpoint of free energy, this is usually prohibitively expensive. Instead of the fissure’s stress being eased by slipping, the crack continues to expand, eventually fracturing.
Brittle materials like ceramics tend to show very low elongation because they do not plastically deform.
Special Alloys
Special alloys are considered as such because they are created for specific uses. These are typically very high in strength and corrosion resistance and maintain these characteristics even at elevated temperatures.

All alloys formed of weak metals are brittle; those made with malleable metals are in some cases ductile. When the proportions are nearly equal, there are as many alloys brittle as flexible; but when one of the metals is in excess, they are most commonly ductile. In combining ductile and brittle metals, the compounds are brittle if the weak metal exceeds, or nearly equals, the proportion of the ductile one. But when the malleable metal greatly exceeds the brittle one, the alloys are usually ductile.
Take, for example of brittle material, iron. It’s solid, but it’s also fragile and rusts rapidly in moist environments.
Fracture Type
Depending on the plasticity of the material, fractures are classified as ductile or brittle. The degree of plastic deformation that a material can bear also influences the type of fracture changes. The fracture is classified as malleable if significant plastic deformation occurs during or before the crack propagation.
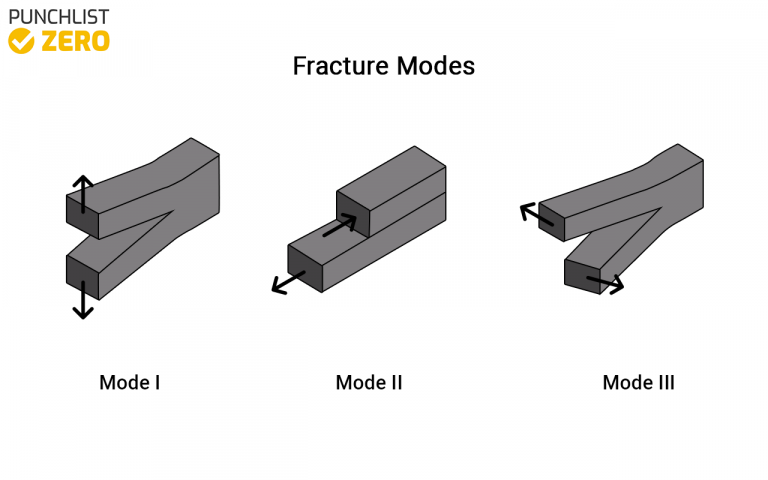
If the only deformation occurs at the micro-level, the fracture refers to as a brittle fracture. Plastic deformation indicates the onset of a fracture. It’s difficult to tell the difference between brittle and ductile fractures, though. This is because several factors influence material deformation. The rate of stress, speed of loading, ambient temperature, and the material’s crystal structure are all factors to consider.
Reducing Unwanted Brittle Fracture

The brittle fracture may be a designed and wanted feature. For instance, safety glass breaks into thousands of pieces upon impact. This brittle fracture reduces the likelihood of fragment embedding into a human.
But in many scenarios brittle fracture is undesirable. For instance, golf clubs are known to experience face fractures and snapped shafts, particularly during cold temperatures.
During both service and testing, it’s critical to keep materials running at or above their Ductile-to-Brittle Transition Temperature (DBTT) to limit the danger of brittle fracture. Similarly, taking steps to identify and locate faults that could weaken the material while in operation or during pressure testing will lessen the risk of brittle fracture during repairs.