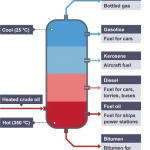
At atmospheric pressure, motor oil has a lower end boiling point of 250°F and up to 700°F. This wide range is due to the variety of different blends of motor oil.

Engineers specify various chemicals for the lubrication of internal combustion engines and known as motor oil, engine oil, or engine lubricant. They typically consist of base oils enhanced with various additives, particularly antiwear additives, detergents, dispersants, and, for multi-grade oils, viscosity index improvers. The primary purpose of motor oil is to reduce friction and wear on moving parts and clean the engine of sludge and varnish. It also enhances piston ring sealing, neutralizes acids produced by gasoline and lubricant oxidation, and cools the engine by removing heat from moving parts.
In this article, you will learn about motor oil types, how motor oil type influences the boiling point, and what happens if motor oil boils.
Motor Oil Types
Motor oil can break into four categories: full synthetic oil, synthetic blends, high mileage oil, and conventional oil.
Full Synthetic Oil

Synthetic oil is a man-made lubricant made up of synthesized chemical substances. Chemically modified materials, such as petroleum components, make up synthetic oils. However, distilled crude oil nearly always serves as the base material. Manufacturers chemically developed this oil to provide a purer, higher-performing oil that performs better at high and low temperatures. Full synthetic motor oil is entirely factory-made.
Synthetic Blend Motor Oil

Manufacturers chemically produce this motor oil to provide a purer, higher-performing oil that operates better at high and low temperatures. It is composed of synthetic and organic oil. Synthetic blend motor oil also gives better gas mileage while protecting automobiles from oxidation and freezing temperatures. Finally, it is far less expensive than synthetic oil.
According to an Automotive Oil Change Association survey, more than half of car owners choose synthetics or synthetic mixes when getting their oil change.
High Mileage Oil

This upgraded type of motor oil includes special additives to reduce oil burn-off and leaks in newer autos. Seal conditioners and additives in high-mileage oils cause o-rings, gaskets, and seals to expand and subsequently reduce seepage. As a result, high mileage oil may reduce the amount of required oil additions and changes. Many high-mileage motor oils have detergents in them and claim to be able to remove sludge from engines.
Conventional Oil

Conventional oil derives from refined crude oil that meets the requirements for lubricating your engine. However, because it generates through a natural process, conventional oil has certain intrinsic inconsistencies in its molecular structure. A wide range of viscosity ratings and quality standards are available in conventional motor oil. It best suits cars with simple engine architectures and drivers that drive conservatively.
How Does Motor Oil Type Influence the Boiling Point?
There can be a massive variance in boiling points. It depends on the type, base stock, additives, and weight. Motor oil typically operates around from 230 to 300 degrees Fahrenheit but can climb to as high as 500+ degrees Fahrenheit for high-performance applications. It’s important that engineers design oil to operate in the proper range. If the oil is colder than its design, it will be too viscous and not move properly. If the oil is hotter than its design, it will be too thin or even worse, suffer thermal degradation.
The reason why motor oils have a high boiling point is that motor oil molecules are incredibly long. Due to its extended size, the larger the molecules, the stronger the dispersion forces the long molecules, leading to a higher boiling point.
As a rule of thumb, as molecules (of similar composition) get more prominent, the boiling point increases. This is basically because larger molecules have larger surface areas and thus more significant places at which intermolecular bonds can form. More bonds mean a more attractive force between two molecules.
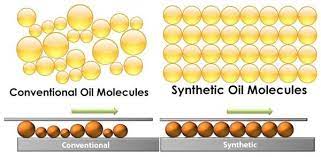
For example, synthetic oil is a lubricant made up of chemical compounds created by breaking down and rebuilding petroleum molecules. A drop of synthetic oil under a microscope reveals millions of virtually identical molecules in size and shape. On the other hand, mineral or traditional oil is refined crude oil. A microscope image of conventional oil reveals millions of molecules of various forms, sizes, and configurations.
What happens if the motor oil boils?

In theory, oil boils like water, but in reality, that should never happen in a motor. When the oil is boiling, it quickly breaks down and loses its intended properties. Viscosity no longer meets the required numbers. As a result, its ability to lubricate is significantly reduced, eventually damaging or seizing the engine.
This type of motor oil damage is frequently referred to as thermal breakdown and must be avoided at all costs.