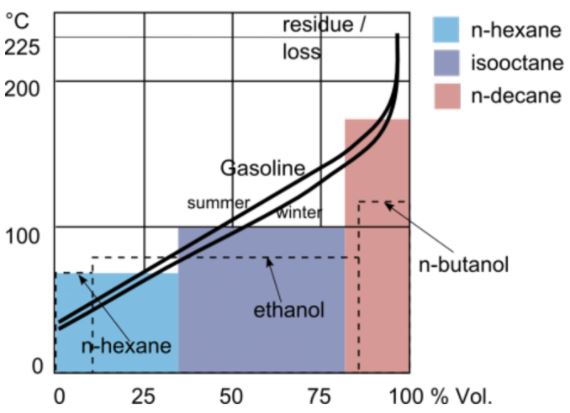
At atmospheric pressure, gasoline has an initial boiling point of 95 °F (35 °C) and a final boiling point of 395 °F (200 °C). This wide range is due to its variety of blends which alter its boiling point value. Also, pressure is another factor that alters gasoline’s boiling point. In this article, you will learn the blends and compounds in gasoline, how they affect its boiling point, and pressure’s effect on gasoline’s boiling point.
Blends and Compounds of Gasoline
Gasoline is one of the products of petroleum refining via fractional distillation. In its raw state, it ignites early upon compression, hence causing knocking in combustion engines. For this reason, it blends with chemical additives to improve gasoline’s ability to undergo significant compression without igniting. The subsequent value that measures its anti-knock robustness is known as octane rating.
In addition, engineers manipulate other characteristics of gasoline such as chemical stability, fuel system cleaning, corrosiveness, and performance by introducing chemicals to its mixture during production. Consequently, a variety of gasoline blends exist, designed to suit various applications. In recent times, the major driver of gasoline blends are environmental regulations, vapor pressure/boiling point, and performance requirements.
Some compounds which are common in gasoline blends include:
- Antiknock Agents: Until recently, Tetraethyllead (TEL) and lead replacement petrol (LRP) were additives to the gasoline blend to help prevent knocking. Due to pollution issues from these compounds, they have been replaced with Methylcyclopentadienyl manganese tricarbonyl (MMT) and oxygen compounds, which have lesser impact on the ozone layer.
- Antioxidants: During long-term storage, the oxidation of alkenes in gasoline leads to its degradation and formation of sticky resin deposits. We use Amines and phenylenediamines to prevent this.
- Detergents: Carbon build up in engines could hamper combustion and easy starting, especially in cold climates. Alkylamines and Alkyl phosphates at the level of 50 ppm to 100 ppm are common detergents in the gasoline blend.
Classification of Blends by Season
It is common to refer to gasoline as ‘gas’ in short. However, gas stores and transfers in liquid form. But in operation, engineers pressurize it to turn to vapor for easier and more efficient combustion. The temperature at which vaporization occurs, which is the boiling point, is of interest especially in temperate regions of the world. Following this, an important concept to consider is a gasoline blend’s Reid Vapor Pressure (RVP). RVP measurs the volatility of a blend. As a result, the higher a blend’s RVP, the more volatile.
Summer Blend and Gasoline Boiling Point
In the summer ambient temperatures are higher. If the surrounding temperature is higher than the gasoline blend boiling point, then it evaporates, thus adding to air pollution. Therefore, in the summer, refiners produce gasoline blends that are less volatile with a much higher boiling point than the prevalent ambient temperature.
Typically, a summer blend consists of 40% FCC gas, 25% straight run gas, 18% reformate, 15% alkylate, and 2% butane. The key ingredient is butane because its fraction in the mixture is what alters the RVP of the blend. In essence, the lower the butane component in the blend, the lower its RVP. On the contrary, its boiling point increases.
In addition to pollution concerns, the efficient operation of gasoline engines is also a driver of the summer blend. The design of fuel pumps is to work with liquid. Hence, if the blend is excessively volatile, the pump becomes unable to push the vapor properly and starves the engine of fuel. This effect, vapor lock, is common in engine-mounted fuel pumps. Pumps located in the fuel tank are more resistant to it.
Winter Blend and Gasoline Boiling Point
As the winter gets cooler, the ideal gasoline blend changes. In particular, increasing amount of butane increases the blend’s RVP. For example, a 10% butane content in the blend contributes 5.2 psi to the mixture. This is how refiners alter the winter blend to make it more volatile. Otherwise, the gasoline engine will start hard and run rough as gasoline will not evaporate sufficiently at cooler temperatures. Therefore, winter blends have lower boiling points than summer blends.
Furthermore, butane is the cheapest component of the gasoline blend. Hence, as its fraction in the mixture increases, the price of the blend reduces. This plays a factor in varying gas prices in temperate regions across the year.
Classification of Gasoline Blends by Octane Rating
Another requirement of gasoline is that it should burn smoothly in an engine without premature detonation, referred to as knocking. Thus, the measure of a blend’s ability to resist knocking when ignited is its octane rating or number. The addition of oxygenates such as ethanol, methyl tert-butyl ether (MTBE), tertiary butyl formate (TBF), and tertiary butyl alcohol (TBA) increases the octane rating of gasoline. Blends with high octane numbers are desirable in new model vehicles and provide high-efficiency combustion for applications such as race cars.
In countries such as the USA, there are three common blends in terms of the octane rating.
- Regular: We refer to this as unleaded gasoline also. It has an octane rating of 87.
- Midgrade: This has a rating between 88 and 90.
- Premium: The octane rating for this blend lies between 91 and 93.

Effect of Pressure on Gasoline Boiling Point
Earlier sections highlight that the relationship between vapor pressure and the boiling point of gasoline is indirectly proportional. The constituents of the blend and their respective fractions influence the value of the vapor pressure. If you alter the proportion of one additive, there is a resultant change in the vapor pressure of the blend. Therefore, gasoline has such a wide temperature range of boiling points.
It is important to note that the vapor pressure is a measure of the pressure exerted by the gas content of the fuel. More volatile blends, which have low boiling points, produce more gas and likewise, more vapor pressure. On the other hand, less volatile blends with higher boiling points produce less gas, thus resulting in lower vapor pressure. In some countries, specific limits are put on the vapor pressure values according to blend to limit pollution levels.